Carbide compounds in molybdenum alloyed steels
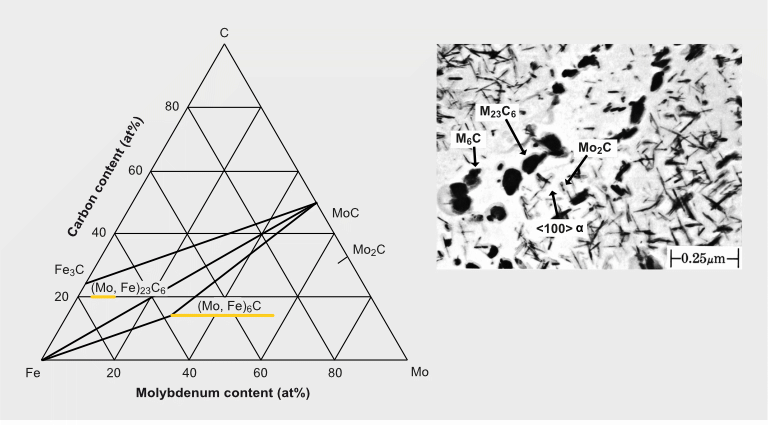
Types of Mo-containing precipitates in a 0.2%C-0.5%Mo steel after quenching and tempering for 16h at 700°C
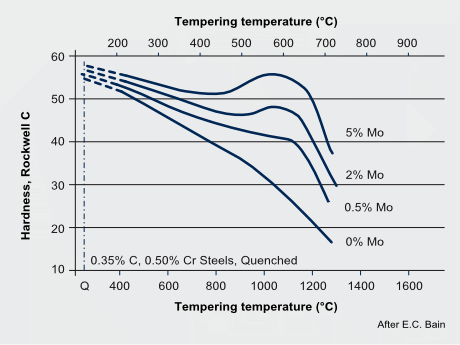
The precipitation of Mo2C particles strongly depends on the carbon and molybdenum content in the steel. It typically occurs when tempering in the temperature range of 600 to 690 °C. For extra-low carbon (<0.05% C) content as used for modern pipeline and automotive HSLA steels, Mo2C precipitation is not expected. Generally, also for lower molybdenum alloy content (<0.3% Mo) Mo2C precipitation is unlikely to occur. In higher Mo-alloyed steels Mo2C precipitation competes with cementite formation and in applicable cases with the precipitation of stronger carbide formers (Ti, Nb, V). Under favorable conditions, the precipitation of Mo2C nano-sized particles contributes to secondary hardening. The strength contribution by Mo2C precipitates can reach values of around 200 MPa.
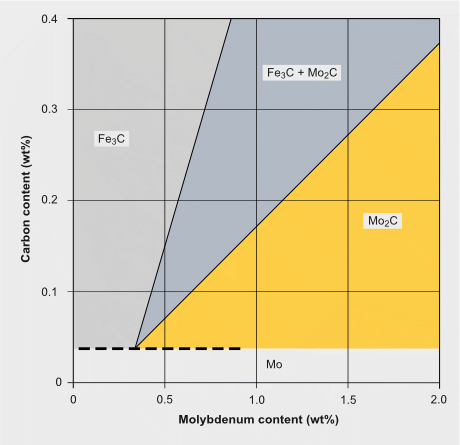
Estimation of carbide formation in Mo-alloyed carbon steels
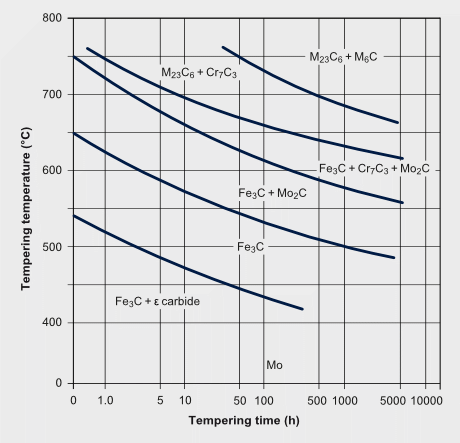
Temper carbide formation sequence in a 2¼Cr-1Mo steel
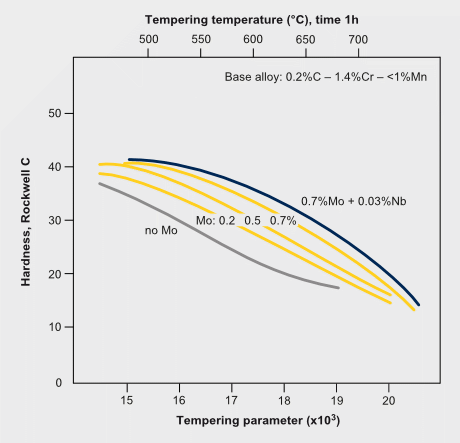
The secondary hardening effect of molybdenum during tempering adds to the solute effect retaining part of the dislocation density. The effect of secondary hardening on tempering is an important function of molybdenum in high speed steels and in some tool- and die steels.
Tempering resistance and secondary hardening in Mo-alloyed carbon steels
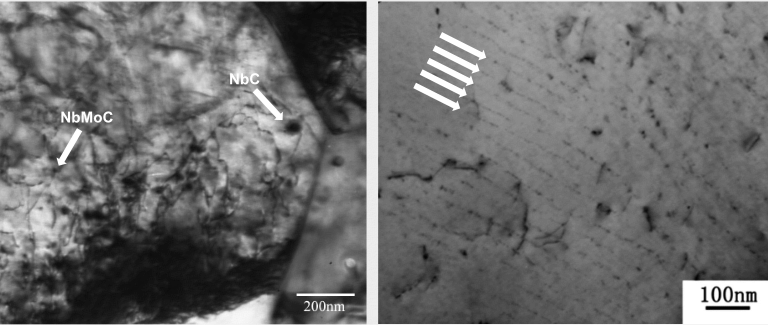
In microalloyed (Nb, Ti, V) steels, Mo participates as a minority fraction in MC type carbides. Often Mo is involved as a precursor phase in the nucleation of such precipitates thereby accelerating the precipitation kinetics. Furthermore, Mo atoms are found segregating at the periphery of nano-sized MC particles limiting their growth rate and enhancing the thermal particle stability. The latter effect is actively used in fire-resistant steels retaining their strength for a longer time period at temperature. So-called nano-Hiten steels take benefit from the nucleation accelerating and growth limitation effects of Mo in conjunction with maximized TiC or NbC precipitation.
Mixed MC-type nano-carbides and interphase precipitation in nano-Hiten steel.
In heat resistant steels molybdenum forms intermetallic Laves phases of the type Fe2(M, Mo) in which M can represent W or Cr. The elevated-temperature and rupture strengths and creep resistance increase with the quantity of Fe2(M, Mo) particles and thus, the molybdenum content. While the 9 Cr-1 Mo steel is martensitic strengthened by carbide precipitates, 9 Cr-2 Mo and 9 Cr-3 Mo steels benefit from the precipitation of Laves phase. Laves phase particles typically appear at high-angle boundaries (PAGBs or martensite block/packet boundaries) with sizes distributed in the range of 50-500 nm. Such particles can form in situ during service or upon a prior isothermal ageing treatment.






