Solute effects of Molybdenum
The molybdenum atom has a considerably larger size than the iron atom. When solute in austenite the mismatch is around 25% while in ferrite it is nearly 10%. Solutes with larger atomic radius induce higher concentration of vacancies. Increasing atomic size mismatch enhances the activation energy for the diffusion of transition metal solutes in the iron lattice. Thus, molybdenum diffusivity in iron is considerably larger than the self−diffusivity of iron. For the range of molybdenum alloy additions in carbon steels, the element is fully soluble in austenite contrary to microalloying elements. Accordingly, metallurgical functionalities of molybdenum during austenite conditioning (rolling, forging) are related to solute drag effects. This reflects in a lower mobility of dislocations and grain boundaries. Consequently, static as well as dynamic recrystallization are strongly retarded resulting in a deformed (non-polygonal) austenite microstructure under suitable hot working conditions.
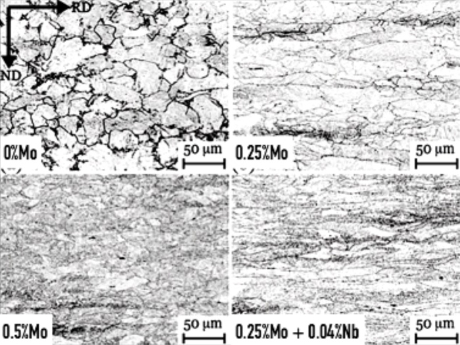
Austenite conditioning by Mo-related solute drag (0.16 %C steel, FRT = 900°C)Prior austenite grain structures after direct quenching from FRT 900 °C (base alloy: 0.16%C, 0.2%Si, 1.1%Mn, 0.5%Cr, 0.5%Ni)
Molybdenum solute in austenite has the effect of lowering the activity of carbon. That again increases the solubility of microalloying elements such as niobium and titanium, which both are strong carbide formers. Hence, with molybdenum alloying soaking treatments of microalloyed steels can be operated at somewhat lower temperature. Furthermore, molybdenum delays strain-induced precipitation in microalloyed steels during TMCP treatments at lower austenite temperature. In that way, a higher share of microalloy species is preserved in solution for later precipitation in ferrite.
Although molybdenum principally has the potential of precipitating in ferrite, still a substantial amount remains solute in most of the relevant alloy concepts. Hence, solute drag effects play a prominent role in ferrite as well. Under typical annealing conditions solute molybdenum retards recovery and recrystallization of priorly cold worked steel. This effect also applies to the subcritical heat affected zone (HAZ) during a welding cycle so that molybdenum alloying counteracts unwanted HAZ softening. Based on the same mechanisms, heat-resistant ferritic steels are typically alloyed with molybdenum, providing sufficient creep strength up to 500 °C operating temperature.
Solute drag effects of various elements on the mobility of ?/? transformation interface and dislocations.
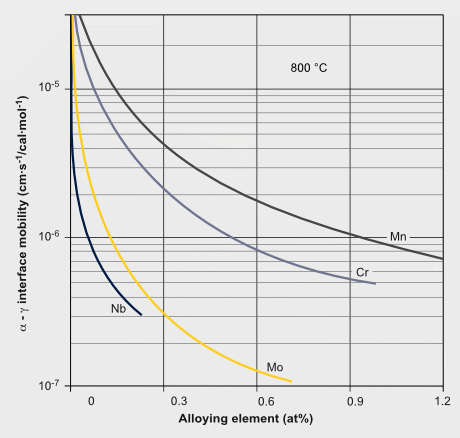
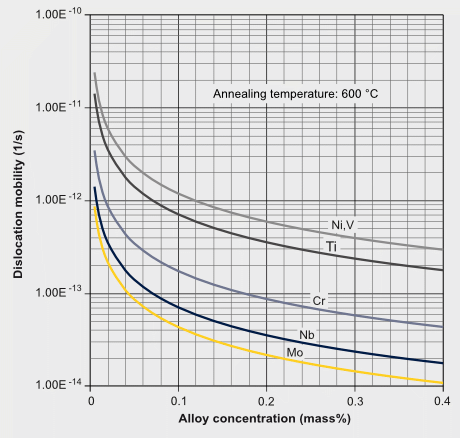
Ref.: S. Yoshida, T. Okumura, H. Kita, J. Takahashi and K. UshiodaMaterials Transactions, Vol. 55, No. 6 (2014) pp. 899 to 906
