Molybdenum Chemical LCI
The molybdenum chemicals covered by this LCI include:
- Ammonium dimolybdate
- Ammonium heptamolybdate
- Pure molybdenum trioxide (both via sublimation and chemical routes)
- Ammonium octamolybdate
- Molybdenum disulphide
- Polymolybdate
- Sodium molybdate dihydrate
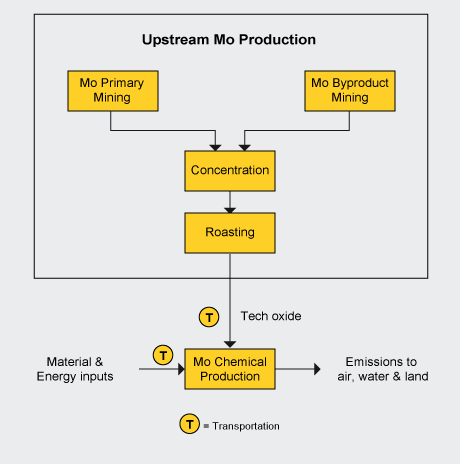
The LCIs are cradle-to-gate, encompassing the processes that include extracting resources from the earth through the point at which the chemicals are ready for shipment to customers. Transportation of the finished product from the shipping dock to a customer is not accounted for. Packaging of the products is included.
The LCIs are based on current data and technologies on the processes, energy and materials consumed, and environmental outputs. Six IMOA member companies participated in this study, with data representing over 90% of western world production of molybdenum chemicals. Data came from sites spanning three continents - Europe, North America, and South America – and a typical range of operating configurations were included and averaged on a weighted basis.
This level of coverage, coupled with robust upstream data on the molybdenum-related inputs, makes IMOA’s chemical LCI study one of the most representative LCI studies carried out for these chemicals and provides a sound basis for LCA studies related to molybdenum chemicals and their applications.
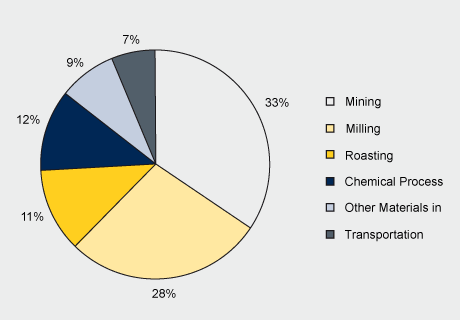
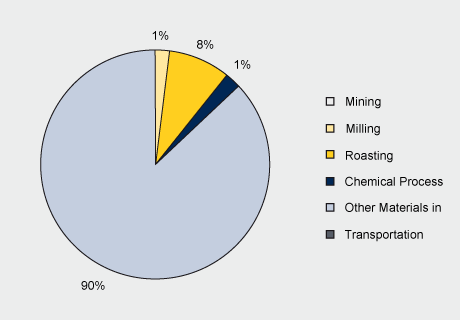
The chemical LCIs drew upon the Mo Metallurgical Study for the upstream intermediate products production. Upstream molybdenum products production plus the chemical process(es) and production of materials and energy used in the chemical production processes, shown in the figure below, make up the system boundaries.