Anti-embrittlement effects of Molybdenum
Ultra-high strength steels usually comprise a martensitic microstructure. The martensitic substructure develops within the prior austenite grain boundary (PAGB) which represents a large-angle boundary. Therefore, impurities tend to preferably segregate at the PAGB. Impurity atoms have the negative effect of reducing the grain boundary cohesion, in other words relatively low stress levels suffice to separate the material along the boundary with brittle fracture character before yielding commences. This phenomenon of boundary embrittlement is observed in as-quenched state but also after tempering treatment.
Temper embrittlement
Temper embrittlement may occur when steels are slowly cooled after tempering through the temperature range between 450 and 550°C. This is due to the segregation of impurities such as phosphorus, arsenic, antimony, and tin on the grain boundaries. The molybdenum atom is comparably large relative to other alloying elements and impurities. It effectively impedes the migration of those elements and thereby provides resistance to temper embrittlement. Another effect is related to molybdenum atoms segregating themselves at the boundary with the effect of enhancing the boundary cohesion, thus opposing the negative effect of impurities. Impurity segregation occurs by diffusion and requires time and elevated temperature. Thus, quenching the steel after tempering can avoid temper embrittlement. However, quenching after tempering is often technically not possible. In that case alloying of already a fairly small amount of molybdenum solves the problem. Taking the ductile-to-brittle transition temperature (DBTT) in a Charpy impact test as a criterion indicates that the embrittlement can be completely suppressed by Mo alloying.
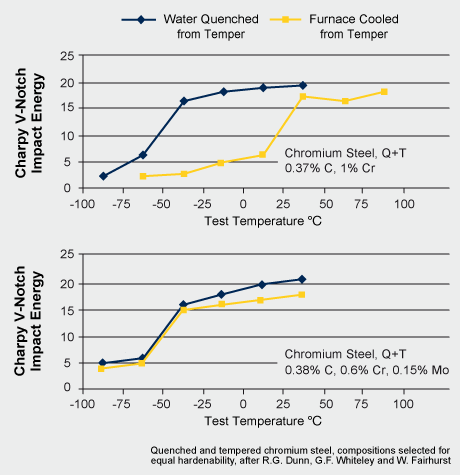
Ductile to brittle transition for two tempered steels, as a function of cooling rate after tempering (after Dunn et al)
Hydrogen embrittlement
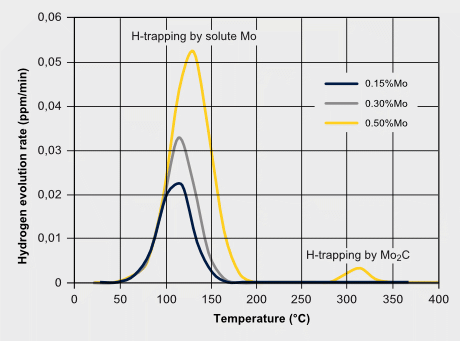
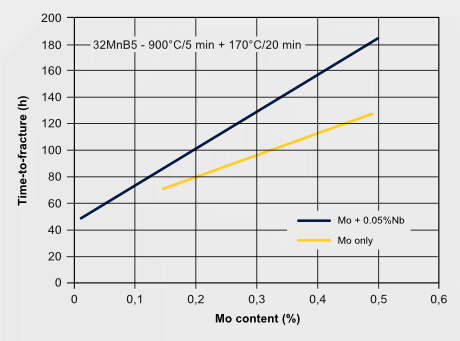
Martensitic steels are also sensitive to hydrogen embrittlement especially when the strength level exceeds 1000 MPa. Hydrogen can exist either as a residual impurity resulting from steel processing, or it is absorbed into the steel during service by corrosive reactions at the steel surface. Hydrogen atoms are very mobile in the iron lattice and tend accumulating at PAGBs or other lattice defect sites. Molybdenum atoms solute in the iron lattice by their large atom size and forthcoming local lattice distortion can act as an atomic hydrogen trap, thus reducing the mobility of hydrogen atoms. This impedes the quick aggregation of hydrogen at critical defect sites. The precipitation of Mo2C in steels with higher molybdenum additions can also provide particle trapping sites for hydrogen. A strong beneficial effect of Mo alloying to hydrogen embrittlement resistance is attributed to the synergistic effect of increasing Mo intergranular segregation and reducing H segregation. First-principles calculations revealed that Mo segregation suppresses H segregation at the PAGB due to the repulsive chemical interaction between Mo and H atoms. The embrittlement severity can be verified by the so-called delayed cracking test where a notched tensile specimen after or during hydrogen charging is subjected to a constant load. For the resultant stress at the notch, the time to fracture is measured as a benchmarking criterion.
H-trapping effects by molybdenum; Enhancing hydrogen embrittlement resistance by Mo-alloying
Sulphide stress cracking (SSC)
In cases, where hydrogen sulphide is the source of the hydrogen the phenomenon is called sulphide stress cracking (SCC). The capability of molybdenum to provide resistance to sulphide stress cracking has been the key to the development of a broad range of steel grades used for Oil Country Tubular Goods (OCTG) and for pressure vessels in chemical and petrochemical plants.
High temperature hydrogen attack
Carbon steel has severe limitations at conditions of hydrogen attack above about 200 °C as are common in processes such as petroleum distilling and catalytic reforming. The hydrogen diffusing into the steel reacts with carbon to form methane and other products. The result is first decarburization of the steel matrix and subsequently fissuring due to high internal gas pressure at localized sites. So called 2¼Cr-1Mo steel with an alloy content of 2.25%Cr and 1% Mo is however resilient to such aggressive conditions for an exposure of at least 500 hours during which the original rupture strength does not deteriorate. The presence of chromium and molybdenum improves the resistance to hydrogen attack for several reasons: both are carbide formers and reduce the activity of nearby carbon atoms and thus the kinetics of methane formation. In addition, molybdenum segregates at grain boundaries reducing the grain boundary energy, thus, impeding the formation of new free surfaces (cracks).
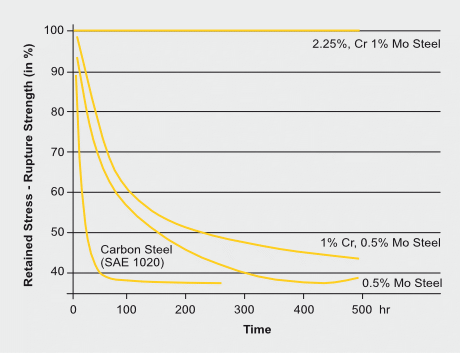
Effect of composition and exposure time on the strength of steels exposed to hydrogen at 63 bar at 540°C. The strength of samples exposed in argon is taken as 100%. (After Nelson)




