Transformation delaying (hardenability) effects
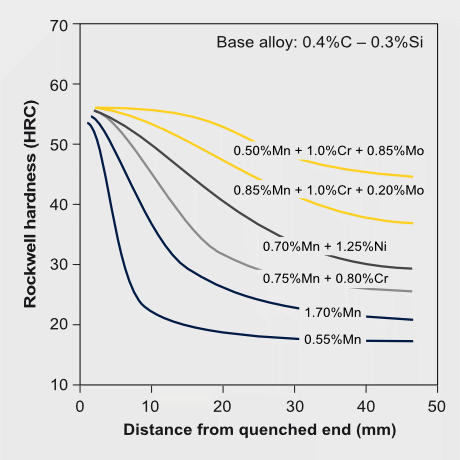
Molybdenum alloying strongly supports hardenability of carbon steels. The relevant physical metallurgical effects relate to solute drag on the phase front between austenite and ferrite as well as on molybdenum reducing the carbon activity. Consequently, the critical cooling rate for achieving transformation into either bainitic or martensitic microstructures is significantly reduced by molybdenum alloying. This greatly facilitates the production of strong carbon steels especially incombination with heavier gages. The hardenable depth of a steel is simulated on laboratory scale by the Jominy end quench test. Such tests indicate that the hardenable depth drastically increases with the molybdenum alloy content saturating at approximately 1%Mo. In fact, molybdenum has the strongest hardening effect amongst the alloying elements in the order: C>Mo>Cr>Mn>Si>Cu>Ni. When alloying combinations of these elements, molybdenum generates synergy effects that can further boost hardenability. In that respect CrMo steels and especially NiCrMo steels are prominent examples.
Hardenability effects and synergies by Mo alloying

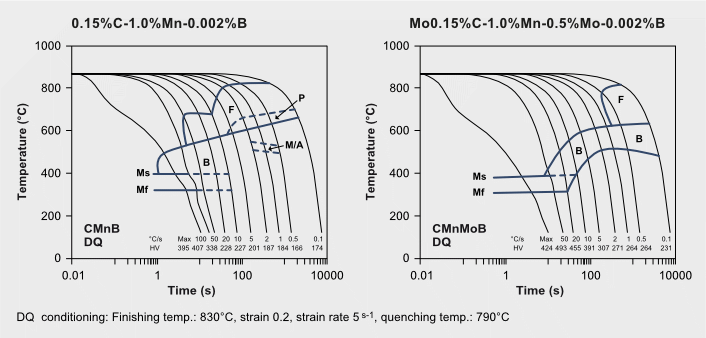
Often boron microalloying is used for enhancing hardenability in carbon steels. Less than 20 weight ppm of boron are needed to achieve a maximum effect. For being effective, boron must be in solution and segregated at the austenite grain boundary. Molybdenum alloying enhances the hardenability efficiency of boron by preventing it from forming iron-boron carbides (Fe23(B,C)6) in the austenite grain boundary and by suppressing dynamic recrystallization of austenite when quenching directly after austenite conditioning (TMCP). Hence, molybdenum alloying is highly relevant in classic reheat-quenched (RQ) and especially in modern direct-quenched (DQ) steels.
Molybdenum’s hardenability synergy with boron in DQ steel