The copper industry advises the use of austenitic stainless steel (i.e. 304/304L, 316/316L) fasteners and structural support components for copper installations, because the metals are relatively close in the galvanic series and the coefficient of thermal expansion is equivalent. Furthermore, stainless steel is more noble and, when used for smaller components, is unlikely to have any significant galvanic effect on the larger copper surface. Stainless steel fasteners are regularly used for other less noble metals.
While galvanic corrosion can lead to failure of the less noble metal, it is also used to extend the life of more noble metals. For example, coatings containing zinc and aluminum are applied to carbon steel because those metals are more anodic and will corrode first, extending the life of the steel.
Design Considerations
If it is not possible to avoid unfavorable dissimilar metal combinations, the best solution is to electrically insulate one from the other. Paint, inert washers and other methods are used. Their expected service life should be assessed relative to the design life of the project and inspections considered. Abrasion caused by differences in the metals’ coefficients of thermal expansion can accelerate inert barrier deterioration. When painted carbon steel and stainless steel are welded together, the welded joint and some of the adjacent stainless steel should be painted.
Dissimilar metal connections are a particular concern when the more anodic metal is structural and has a smaller surface area. If contact occurs at any location, then all of the continuously connected metals are part of the galvanic cell and determine the relative surface area ratio. If a few inert washers between aluminum or carbon steel framing and stainless steel panels are missing, if paint abrades due to differences in coefficients of thermal expansion, or if metal burs cut through thin double-sided adhesive tape, then electrical contact at those connections can weaken the structure as was shown in the Cathedral example. Specifying fasteners with built-in inert washers and similar precautions can reduce problems but may not be a permanent solution.
Stainless steel roofing or façade elements should be supported by stainless steel structural sections, fasteners and clips to achieve maximum service life. Additionally, it is also important to make sure that fasteners connecting multiple metals match the most noble metal.
General guidance for locations with moderate salt exposure and similar surface areas is given in the table. Large differences in surface area ratio will change the corrosion rates. For example, a stainless steel bolt in a large carbon steel plate in a rain shedding application is generally not a concern, but the railing example above shows that the opposite significantly increases corrosion rates. The appropriateness of metal combinations and the risk of corrosion will vary with the specific environmental conditions and design details.
Guidance on the Risk of Galvanic Corrosion in a Marine or Deicing Salt Exposed Atmosphere for Some Common Metals with Similar Surface Areas
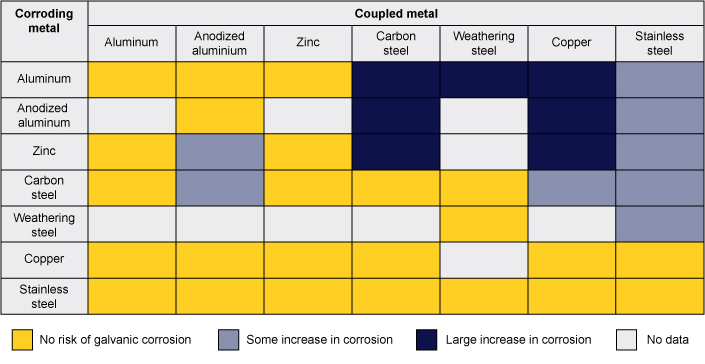
Note: Extracted from Galvanic Corrosion: A Practical Guide for Engineers, Table 7.4, published by NACE International – The Corrosion Society, 2001, author Roger Francis
Some Exceptions & Anomalies
Galvanic corrosion can occur without initial physical contact. For example, localized corrosion of a metal may result in water soluble corrosion products which can deposit on to the surface of another metal. This can cause localized, intense galvanic cells. This is a particular concern with more electropositive metals like copper and it is why downspouts and gutters from a copper roof should always be in copper or stainless steel and not aluminum. Always consider the relative placement of metals on a building.
Not all environments produce the same galvanic results and using the galvanic series for seawater may not always provide reliable predictions. For example, research has shown that galvanic corrosion is not a concern between stainless and carbon steel in concrete. In this alkaline environment, there is more galvanic corrosion between sections of carbon steel that are active (i.e. corroding) and passive (i.e. not corroding) than between stainless and carbon steel.
Other Resources
The Nickel Institute brochure, Guidelines for Corrosion Prevention, provides information on every common type of corrosion and includes data on the relative corrosion rates of metals in different environments. IMOA has a Stainless Steel Selection System with more specific guidance on evaluating exterior service environments and selection of appropriate alloy(s) to avoid atmospheric corrosion. There are also specific pages on avoiding corrosion caused by soil, coastal and deicing salt exposure.
Corrosion engineering is a specialized area within metallurgical engineering and materials science. It can be quite complicated and experts specialize in specific environments such as corrosive water, industrial environments or atmospheric corrosion. If there is uncertainty, the advice of an expert is recommended.




