The use of molybdates as corrosion inhibitors and in paints is summarised in Table 5. Soluble molybdates, e.g. sodium molybdate, are used in solution in central heating systems and motor engine coolants. The insoluble molybdates are applied as paint primers and as paints. Molybdates, unless combined with a coloured cation or mixed with a coloured compound, are white and so have application as white pigments. For example, zinc molybdate which is non-toxic and an excellent corrosion inhibitor. The orange colour of the molybdenum orange pigment is due to the presence of chromate. This pigment consists of lead molybdate, chromate and sulfate in a mixed crystal system. Lead chromate is yellow whereas the molybdates chromes are bright orange. The colour change is due to a modification of the crystal structure of lead chromate when crystallised in the presence of molybdate.
Corrosion inhibiting pigments, primarily zinc molybdate, but also molybdates of calcium and strontium are used commercially in paints. These pigments are white and can be used as a primer or as a tint with any other colour.
Molybdates are non-toxic and are less aggressive oxidants than chromates toward organic additives that may be used in corrosion inhibiting formulations. A prime application is in cooling water in air-conditioning and heating systems to protect mild steel used in their construction.
Molybdates are used to inhibit corrosion in water-based hydraulic systems and in automobile engine anti-freeze.
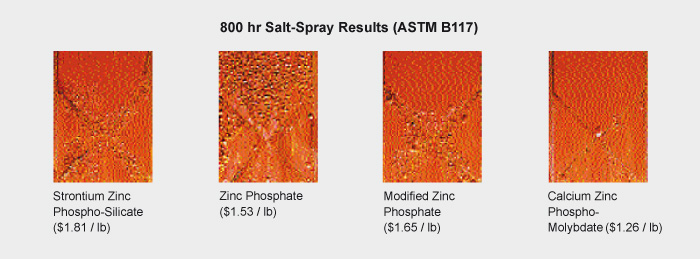
The ability of molybdate to inhibit corrosion is demonstrated in standard tests in which steel plates are exposed to a salt spray. Molybdate is seen to prevent surface pitting of test pieces (e.g., see Fig. 1).