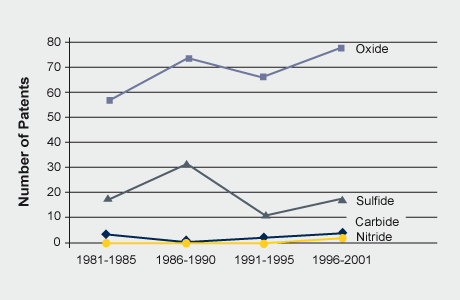
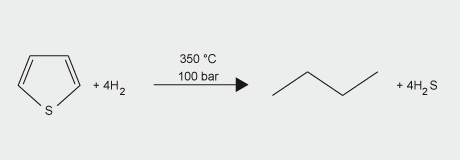
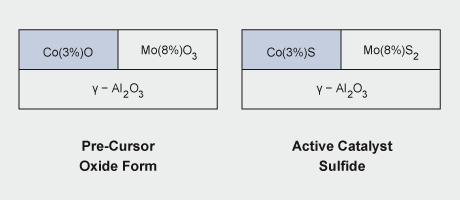
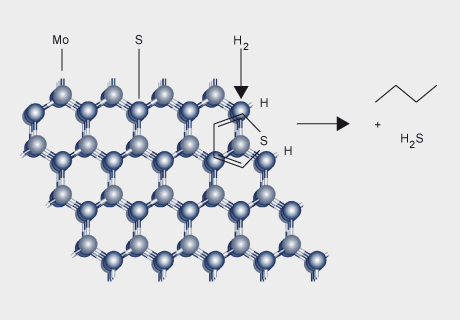
Molybdenum-based catalysts have a number of important applications in the petroleum and plastics industries. A major use is in the hydrodesulfurization (HDS) of petroleum, petrochemicals and coal-derived liquids. The catalyst comprises MoS2 supported on alumina and promoted by cobalt or nickel and is prepared by sulfiding cobalt and molybdenum oxides on alumina. Continued use of sulfur-containing fossil fuels will require molybdenum catalysts for desulfurization. Molybdenum not only allows for economical fuel refining but also contributes to a safer environment through lower sulfur emissions.
Molybdenum catalysts are resistant to poisoning by sulfur and, for example, catalyse conversion of hydrogen and carbon monoxide from the pyrolysis of waste materials to alcohols in the presence of sulfur, under conditions that would poison precious metal catalysts. Similarly Mo-based catalysts have been used in the conversion of coal to hydrocarbon liquids.
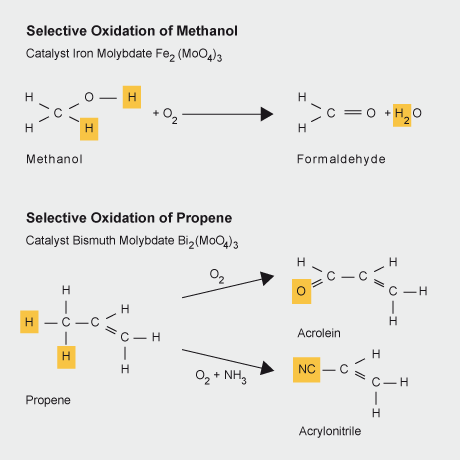
As a component of the bismuth molybdate selective oxidation catalyst molybdenum participates in the selective oxidation of, for example, propene, ammonia, and air to acrylonitrile, acetonitrile and other chemicals which are raw materials for the plastics and fibre industries. Similarly molybdenum in iron molybdate catalyses the selective oxidation of methanol to formaldehyde.