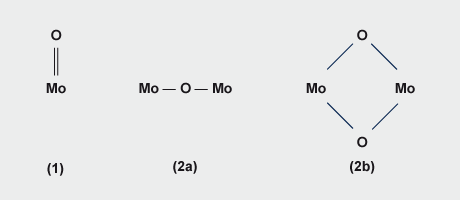
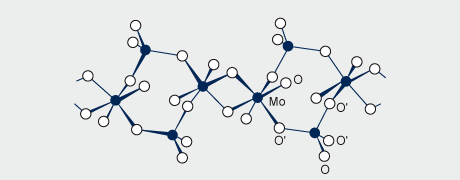
The structure of ammonium dimolybdate (Fig. 3) determined by X-ray crystallography on crystals from the manufacturing plant, consists of infinite chains of pairs of edge-shared MoO6 octahedra, adjacent pairs being linked by MoO4 tetrahedra with the chains having equal numbers of octahedra and tetrahedra. The structure is quite different from that of dichromate which contains discrete [Cr2O7] 2- ions. Ammonium heptamolybdate, (NH4)6Mo7O24.4H2O, is prepared by crystallising a solution of MoO3 in aqueous ammonia of the requisite NH3/Mo/H2O stoichiometry at ambient temperature. The structure comprises linked [MoO6] octahedra only.
If molybdates are to be used in organic media then they are in the form of suspensions although some solubility of molybdates in organic solvents (alcohols and ketones) can be achieved by replacing sodium and ammonium balancing cations with alkylammonium cations. The alkylammonium molybdates are salts - not complexes. The compound tetrakis(isopropylammonium) octamolybdate(VI), [C3H10N]4[Mo8O26] is typical of this type of compound.
Heteropolymolybdates
The heteropolymolybdates consist of [MoO6] octahedra incorporating atoms of a different element, the heteroatom. The heteroatoms are completely surrounded by the oxygen atoms of the [MoO6] octahedra. The resulting coordination of the heteroatom may be tetrahedral or octahedral. The 12-molybdo species,
[X n+MO12O40] (8−n)-, is an important group with tetrahedrally coordinated heteroatoms (X). An example is 12-molybdophosphoric acid H3[PMo12O40] .28 H2O, prepared by dissolving molybdenum trioxide in phosphoric acid; it is yellow and readily soluble in water. Ammonium 12-molybdophosphate, which precipitates when ammonium molybdate is added to a solution of disodium hydrogen phosphate, is used for the gravimetric determination of phosphate.
A number of heteropolymolybdates are soluble in organo-oxygen solvents, e.g. 12-molybdosilicic acid and 12-molybdophosphoric acid.