It has a layer structure with Mo atoms in trigonal prismatic coordination sandwiched between sheets of S atoms. It is unreactive towards water and acids and alkalis. However, molybdenum disulfide and other Mo-S compounds oxidise when heated in air to molybdenum trioxide. Because of the unreactivity of molybdenum disulfide Mo-S compounds are made via reaction with molybdates as in the preparation of hydrodesulfurization catalysts. Molybdenum disulfide is used, per se, as a dry lubricant.
Molybdenum forms complexes with sulfide ([MoS4] 2-) and many organosulfur ligands. These compounds unlike their oxygen counterparts are often brightly coloured, e.g. [MoS4] 2- is deep orange-red. The dithiocarbamates and dithiophosphates are applied as oil soluble lubricant additives.
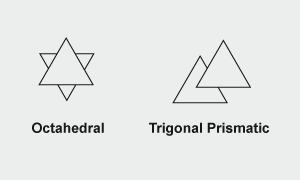
Octahedral and trigonal prismatic structures viewed through triangular faces. A sulfur atom is located at each vertex. A molybdenum atom is located between each pair of triangles. MoO3 and molybdates with linked [MoO6] groups have the octahedral structure; MoS2 is trigonal prismatic.
Two-dimensional molybdenum disulfide (2D-MoS2)
Currently (2023), there is an explosion of interest in the preparation, properties, and applications of two-dimensional molybdenum disulfide. 2D-MoS2 is a form of molybdenum disulfide with separated MoS2 layers a few atoms thick. The thickness of monolayer MoS2 is 0.65 nm. It is a nanomaterial in the class of 2D transition metal dichalcogenides. A nanomaterial is a crystalline substance having at least one dimension in the nanometre scale. When only one dimension is so restricted, we have a layered shape, a two-dimensional (2D) material. Within an individual layer molybdenum and sulfur are strongly covalently bonded; the bonds between adjacent layers (or sheets) are weak (van der Waals interaction). The layers may be separated to form MoS2 flakes or films consisting of one or a few layers.
2D-MoS2 flakes may be prepared by exfoliation (separation of layers) of bulk (three-dimensional) MoS2 or by chemical synthesis, e.g. reaction of MoO3 with H2S.
Because of their unique properties 2D-MoS2 nanosheets have promising applications in, for example:
- Electronic and optoelectronic devices as a direct band gap semiconductor e.g. for transistors, gas sensors, solar energy harvesting
- Water-related environmental applications e.g. contaminant adsorption, photocatalysis, membrane-based separation, sensing, and disinfection
- Energy storage and delivery e.g. batteries
- Medicine e.g. drug delivery, phototherapy, bioimaging, theranostics, and biosensing
For sources and further reading on two-dimensional MoS2 these OPEN ACCESS reviews are suggested:
- Li X, Zhu H. Two-dimensional MoS2: Properties, preparation, and applications. Journal of Materiomics. 2015 Mar 1;1(1):33-44.
- Bazaka K, Levchenko I, Lim JW, Baranov O, Corbella C, Xu S, Keidar M. MoS2-based nanostructures: synthesis and applications in medicine. Journal of Physics D: Applied Physics. 2019 Feb 27;52(18):183001.
- Kumar VP, Panda DK. Next generation 2D material molybdenum disulfide (MoS2): properties, applications and challenges. ECS Journal of Solid State Science and Technology. 2022 Mar 25;11(3):033012.

